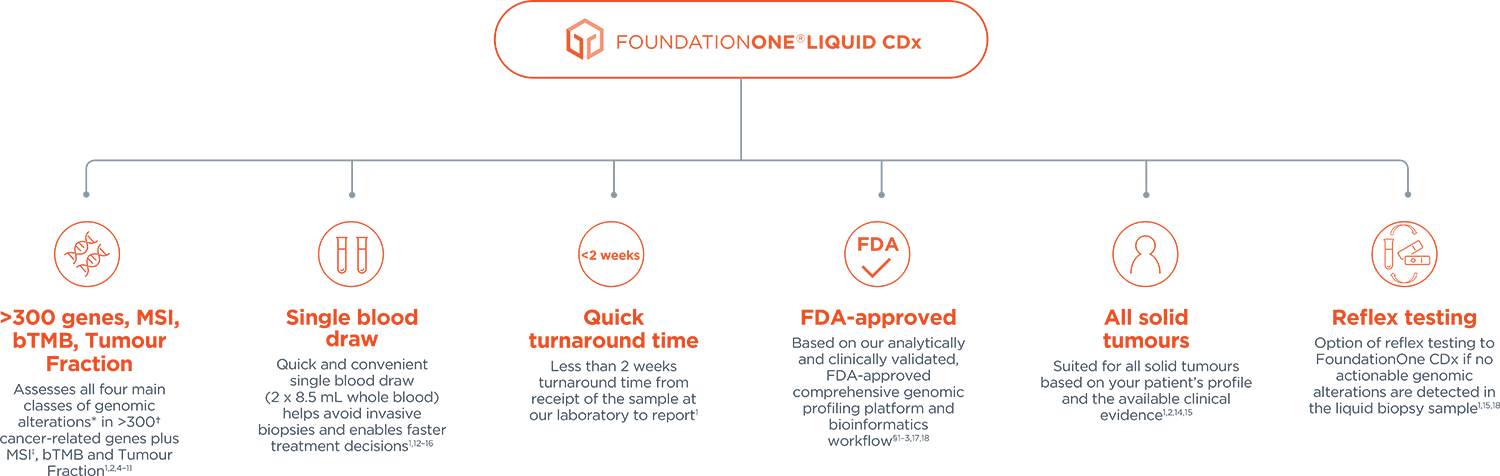
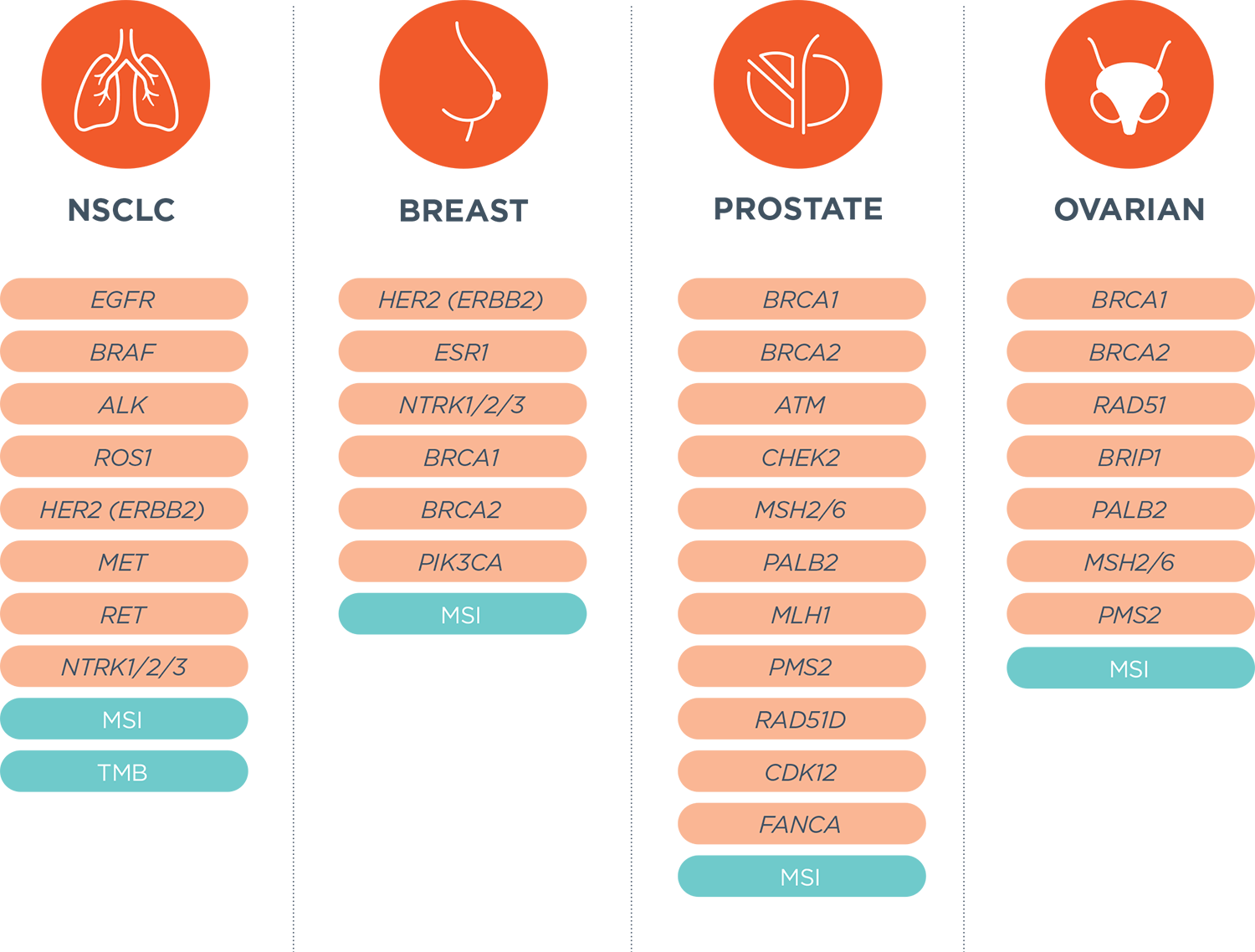
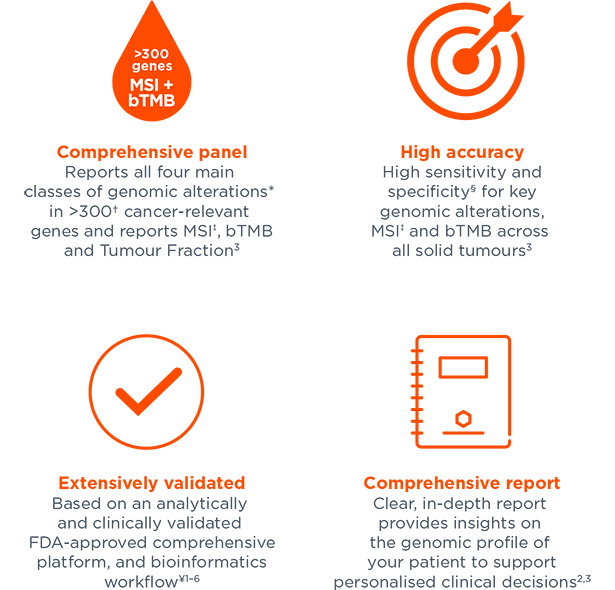
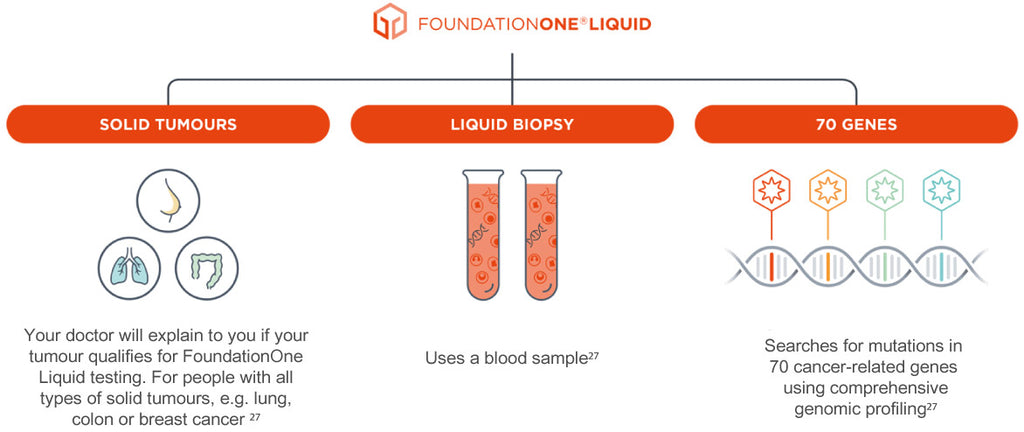
Foundation Medicine on LinkedIn: Join Foundation Medicine's Lucas Dennis, VP of Franchise Development, for…

Foundation Medicine to launch liquid biopsy companion diagnostic following FDA approval - MedCity News
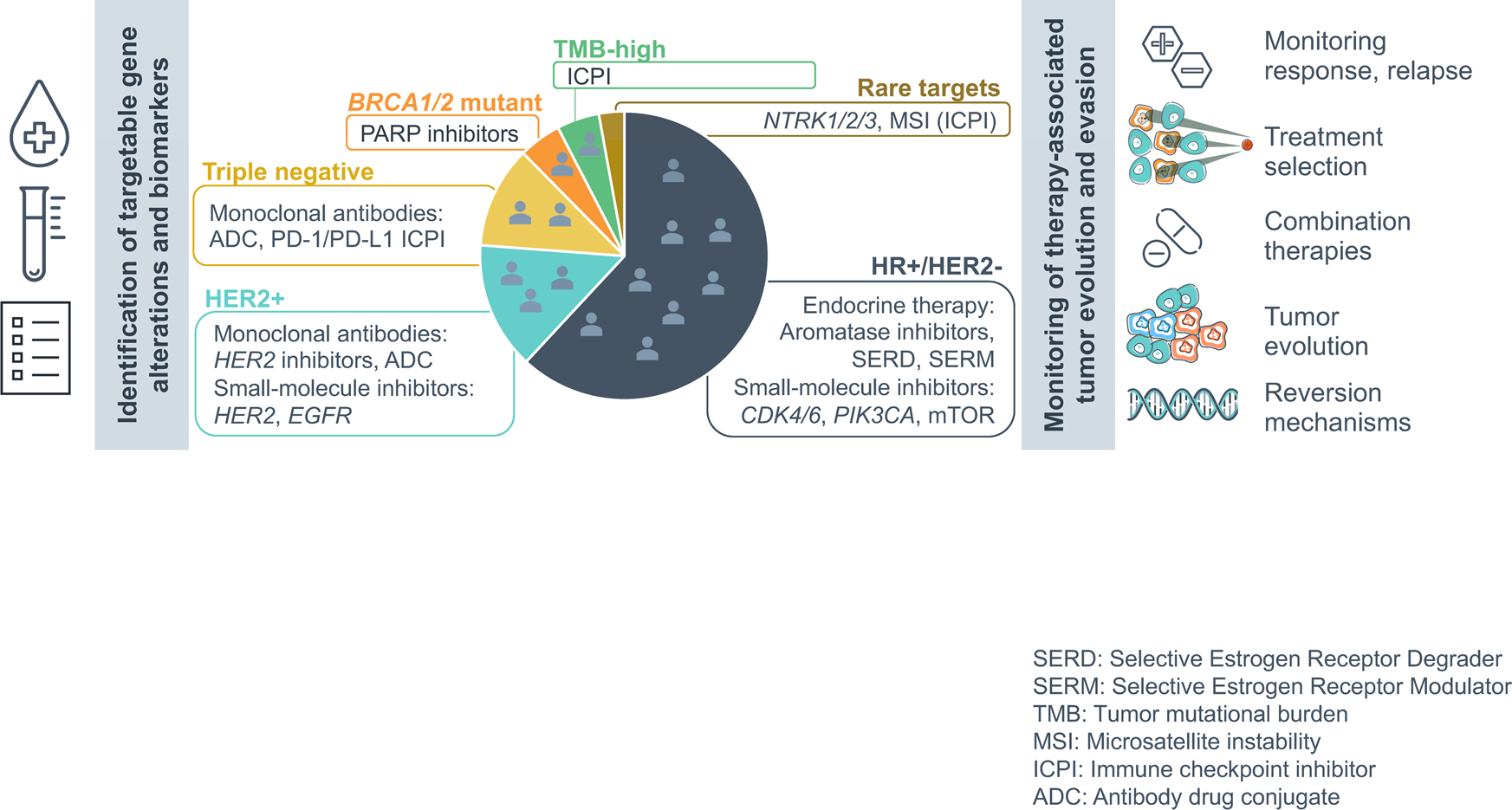
Tissue and liquid biopsy profiling reveal convergent tumor evolution and therapy evasion in breast cancer | Nature Communications

Comprehensive genomic profiling in oncology – from vision to reality | Latest news for Doctors, Nurses and Pharmacists | Pharmacy